
New Drug Designations - April 2024
Shots:
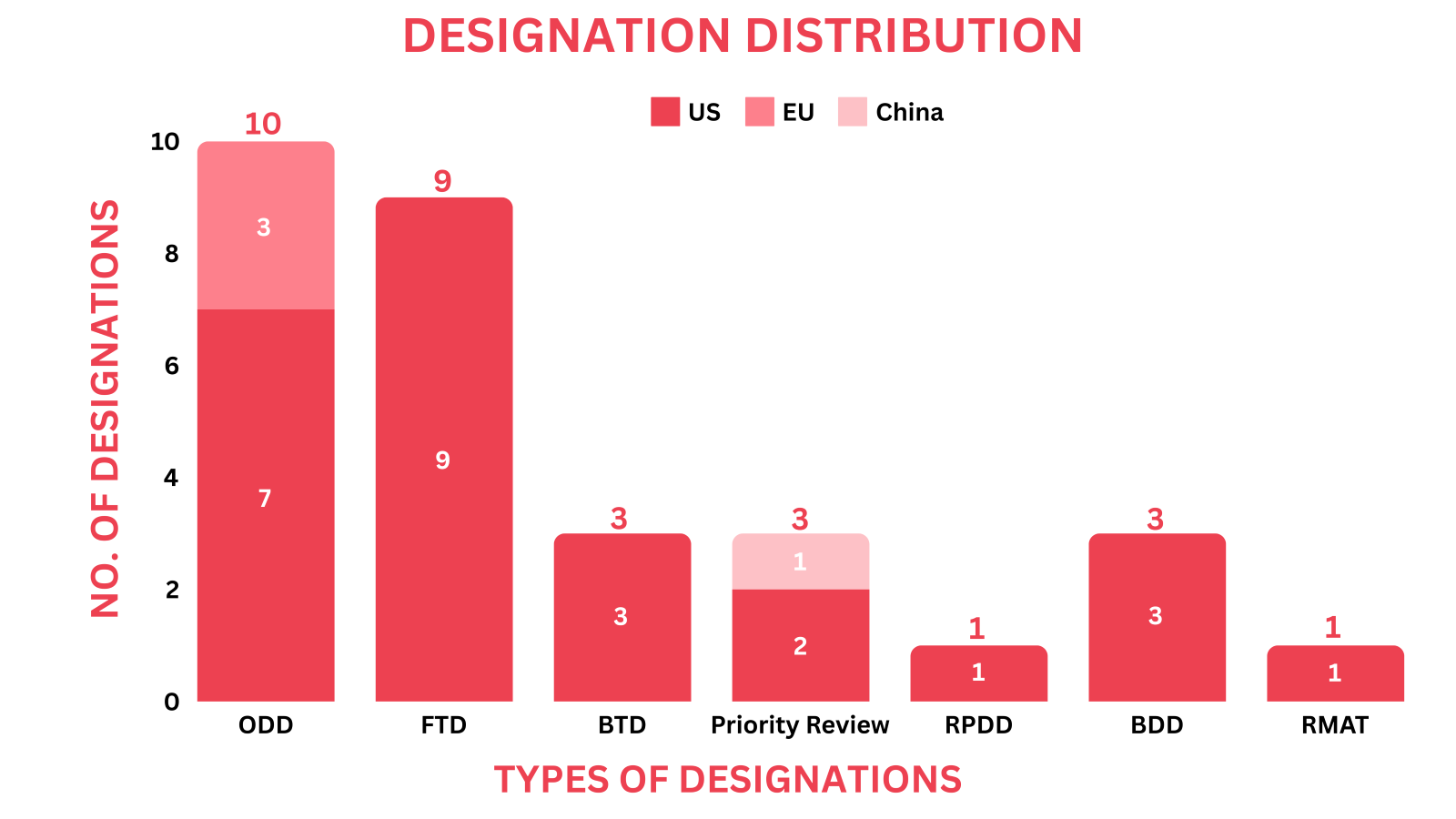
- PharmaShots' designation report provides a concise overview of several drugs and their designations by the FDA, NMPA and EMA. This month’s report includes designations allotted to 11 small molecules, 7 biologics, 7 cell & gene therapies, 1 antiviral, 1 peptide and 3 devices
- Lisata Therapeutics’ LSTA1 received ODD this month and was granted with RPDD in Mar 2024 from the US FDA for the treatment of Osteosarcoma
- PharmaShots has compiled a list of a total of 27 drugs and 3 devices awarded with designations by multiple regulatory bodies in April 2024

Tecarfarin
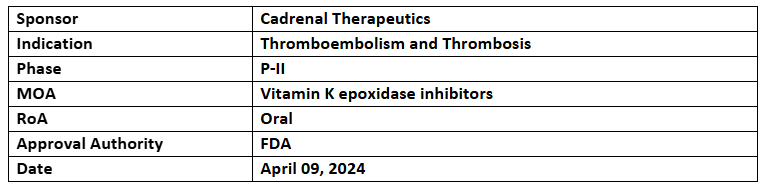
- The US FDA has granted ODD to tecarfarin for preventing thromboembolism and thrombosis in patients who have been implanted with mechanical circulatory support devices incl. LVAD, RVAD, biventricular assist device & total artificial heart
CAN-2409
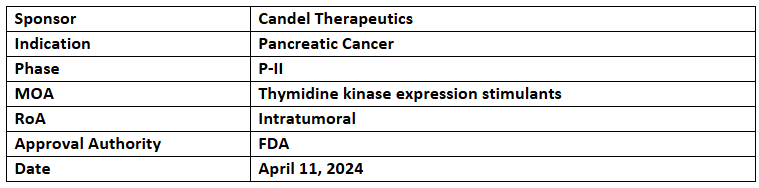
- Candel’s CAN-2409 has received the US FDA’s ODD based on its ongoing P-II trial evaluation in combination with valacyclovir and SoC chemoradiation as a treatment for borderline resectable pancreatic cancer
- As of Mar 2024, demonstrated an improved mOS of 28.8mos. with CAN-2409 vs 12.5mos. in the control arm, a survival rate of 71.4% vs 16.7% was observed in patients post chemoradiation at 24mos. and an estimated survival of 47.6% vs 16.7% was seen at 36mos.
- Further analysis of resected tumors demonstrated anti-tumor response by altering the tumor microenvironment leading to cytotoxic lymphocyte activation and elevated proinflammatory cytokines
- CAN-2409 has been granted Fast Track Designation by the FDA for treatment of PDAC
IGNK001 (Gengluecel)
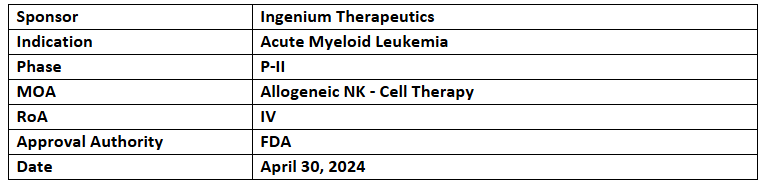
- Ingenium has received MFDS’ approval to initiate P-II study in Korea
- Company plans to initiate study in the US and aims to enroll 80 patients and complete the studies by Dec 2027
NM5072
- The US FDA has granted orphan drug designation to the company’s NM5072, first-in-class monoclonal antibody, for the treatment of paroxysmal nocturnal hemoglobinuria (PNH)
- The drug concluded P-I study in healthy participants (n=48) showing a well-tolerated & favorable safety profile at all dosing up to 20mg/kg and AP inhibition demonstrating a dose-dependent response
- The company anticipates the P-II clinical evaluation of NM5072 in treatment-naïve patients with paroxysmal nocturnal hemoglobinuria and is being reviewed for other indications such as C3G & aHUS. The SC formulation is planned for upcoming trials
LSTA1
- The drug has been previously designated with RPDD for osteosarcoma in Mar 2024
- LSTA1 is developed for the activation of an uptake pathway, enabling the penetration of co-administered or tethered anti-cancer drugs into solid tumors
BLR-200
-
BLR Bio received a $780,000 grant from the Canadian Institutes of Health Research in 2022 to study BLR-200 in scleroderma with the University of Saskatchewan. The research has shown promising in vivo results and is underway
BVX001
- With the designation, the company also concluded Initial Targeted Engagement for Regulatory Advice (INTERACT) meeting with CDER on BVX001's pharmacology, toxicology, and CMC for IND preparation
- The company is advancing BVX001's preclinical study & plans a pre-IND submission, with further efficacy & safety results anticipated at a hematology conference in H2’24
- BVX001 is the company’s new twin antigen (CD33/CD7) that works by targeting bxADC
Annamycin
- The company’s annamycin has also been designated with the US FDA’s FTD & ODD for treating r/r AML plus ODD for treating STS lung mets
- Annamycin, a non-cardiotoxic anthracycline, has demonstrated safety in 82 individuals across the US & EU studies. In a European AML trial, annamycin + cytarabine (MB-106) achieved a 60% complete response rate (CRc) in 2L individuals (n=10) and a 39% CRc overall (n=18). 1 of them maintained a durable complete response for 1yr.
- Enrollment of 2L participants in MB-106 is complete, while 1L & 3L, recruitment continues
- The drug is also being developed for STS lung metastases and other indications
Sevasemten (EDG-5506)
- The drug has previously been designated with the US FDA’s ODD and FTD for Becker and Duchenne as well as RPDD for Duchenne
- The company is assessing sevasemten under P-II (CANYON) study for its safety & efficacy on muscle function and biomarkers in Becker adults. The trial is expanded to include 120 additional adults across a pivotal arm, GRAND CANYON at the US and EU
- Furthermore, the P-II (LYNX) study assessed the safety, PK, biomarkers of muscle damage, and function in DMD boys and the P-II (FOX) study evaluates similar parameters in children and adolescents treated with gene therapy
NXC-201
- The company is currently assessing NXC-201 in the P-Ib/II (NEXICART-1) study for its safety & efficacy in multiple myeloma patients, began in Feb 2021
- As per Davis et al., 2023 Transplantation and Cellular Therapy, commercial CAR-Ts produced mPFS: 6.9 mos. in frail r/r MM patients in a real-world setting. 61% of patients in the dataset were considered frail.
- NXC-201 has been awarded ODD by the US FDA in both AL Amyloidosis and MM, and ODD by the EMA in AL Amyloidosis
- Approval would be based on Centralized authorization procedure and once approved NXC-201 will gain 10yrs market exclusivity from EU
 Pixclara (TLX101-CDx)
Pixclara (TLX101-CDx)

- The US FDA has granted FTD to Pixclara (TLX101-CDx), the company’s imaging agent for glioma
- The FTD is for characterizing progressive or recurrent glioma using PET. Telix is planning the NDA of TLX101-CDx in the US for this indication in both adult and pediatric patients
- Furthermore, Telix has signed an exclusive research & data license agreement with UCSF to jointly develop & commercialize TLX101-CDx in the US, pending regulatory approval
- Telix has chosen PharmaLogic Holdings ss its commercial manufacturing and pharmacy distribution partner for TLX101-CDx across the US
Enibarcimab
- The P-II (AdrenOSS-2) study (n = 301) carried out a prespecified analysis of cDPP3 as a 2nd biomarker (alongside ADM) to remove those patients unlikely to respond enibarcimab
- The study depicted 24% to 8% improvement in organ dysfunction & reduction in day 28 all-cause mortality with enibarcimab
- The company is now planning a P-IIb/III study to verify enibarcimab’s ability to reduce septic shock mortality with precision medicine method
CTX-009
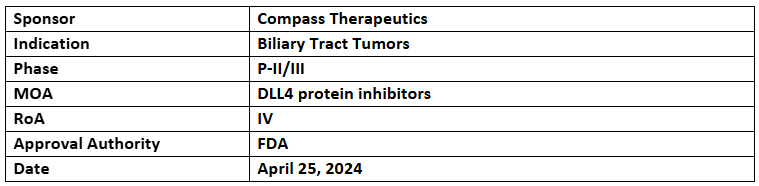
- The US FDA has granted FTD to CTX-009 + Paclitaxel for treating metastatic or locally advanced BTC patients who are treatment experienced
- The drug demonstrated favorable clinical outcomes in P-II study of BTC patients
- The topline results of P-II/III (COMPANION-002) trial, being conducted in the US, are anticipated by YE 2024
Tamibarotene
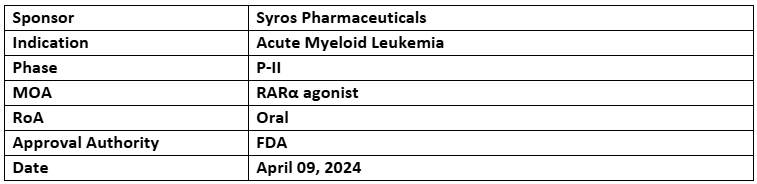
- The US FDA has granted FTD to the combination of tamibarotene + azacitidine & venetoclax for treating newly diagnosed AML with RARA overexpression in adults (75yrs.)
- The P-II (SELECT-AML-1) study assesses the combination of tamibarotene with venetoclax & azacitidine to treat AML with RARA overexpression
- The initial SELECT-AML-1 study results, reported in Dec 2023, showed 100% (9/9) vs 70% (7/10) CR/CRi rate with the combination vs venetoclax & azacitidine alone plus 78% vs 30% CR rates with a rapid median time to CR/CRi response. Additional data is anticipated in H2 2024
- Tamibarotene + azacitidine is also being evaluated in P-III (SELECT-MDS-1) study to treat newly diagnosed higher-risk myelodysplastic syndrome (MDS) with RARA overexpression. Enrollment for the pivotal efficacy analysis was completed in Q1’24, with response data expected by Q4’24. In Jan 2023, the US FDA granted FTD to tamibarotene for this treatment
AB-1002
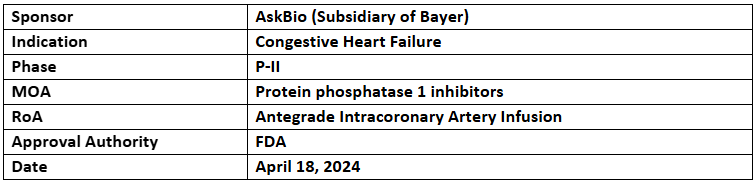
- AB-1002 is being investigated in the P-II (GenePHIT) study for its safety and efficacy in males and females (>18yrs.) with non-ischemic cardiomyopathy and NYHA Class III heart failure symptoms. Patient enrollment is underway
- AB-1002 is an investigational one-time gene therapy to promote production of a protein inhibitor (I-1c) that blocks protein phosphatase 1, leading to therapeutic effects for congestive heart failure (CHF)
LYT-200
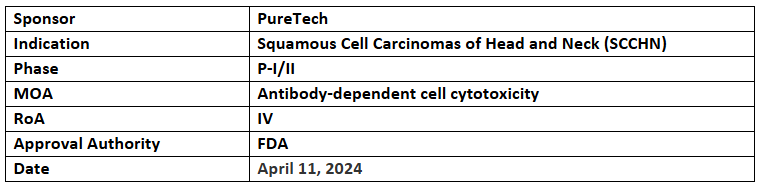
- The US FDA has granted FTD to LYT-200, human IgG4 mAb targeting galectin-9, in addition with tislelizumab to treat recurrent/metastatic SCCHN
- The company’s LYT-200 is under the P-I/II trial as monotx. & combined with tislelizumab for advanced/metastatic solid tumors incl. SCCHN in which it demonstrated disease control, anti-tumor activity & a favorable safety profile across all cohorts
- LYT-200 is also being investigated in the P-Ib study as monotx. & in combination with venetoclax & hypomethylating agents for treating hematological malignancies such as AML & high-risk myelodysplastic syndrome in which it showed a favorable safety & tolerability profile as well as potential clinical activity
- FDA has also granted ODD to LYT-200 for the treatment of AML in Mar2024
LX2006
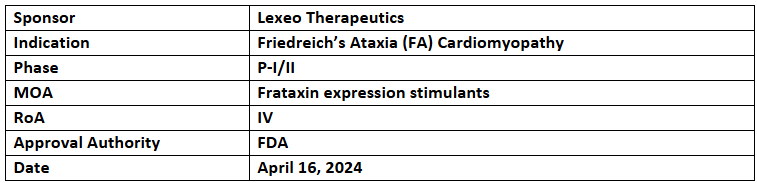
- The US FDA’s FTD for Friedreich’s ataxia (FA) cardiomyopathy was supported by the preclinical results
- LX2006 is presently being assessed in the P-I/II (SUNRISE-FA) for its safety, effectiveness & tolerability in FA cardiomyopathy patients. It is given as a one-time IV infusion in up to three ascending-dose cohorts
- The long-term safety and efficacy will be assessed for 5yrs., incl. 4yrs. of follow-up
- LX2006, AAVrh.10hFXN-based gene therapy, works by transferring a functional frataxin gene, enabling frataxin protein expression to restore mitochondrial function in myocardial cells
DD01
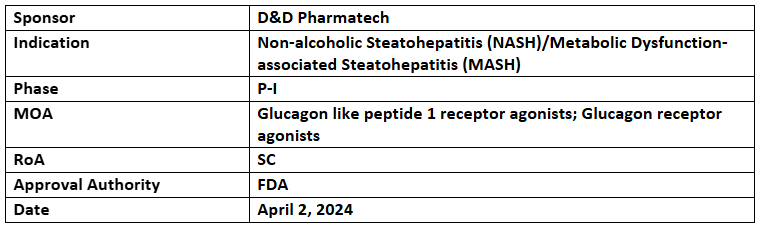
- The FTD was supported by the P-I trial investigating the safety and efficacy of DD01 vs PBO in overweight/obese participants with T2D and metabolic dysfunction-associated fatty liver disease (MAFLD). The company is planning for P-II trial in biopsy-confirmed MASH
- The results showed a >50% reduction in hepatic steatosis at 4wks. and ≥30% reduction in liver fat was observed in 100% of patients
- Animal models showed reduction in hepatic steatosis, lobular inflammation, ballooning & fibrosis with weight loss and improved glycemic control, indicating potential benefits for treating fatty liver disease, obesity and type 2 diabetes patients
PT217
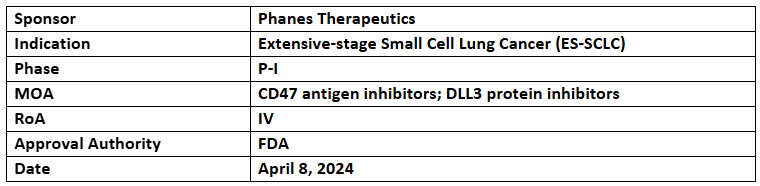
- The US FDA has granted FTD to PT217 for treating ES-SCLC patients with disease progression post Pt-based CT with/without a checkpoint inhibitor. It was designated with the US FDA’s ODD in 2022 for SCLC
- The P-I (SKYBRIDGE) study assesses the safety, tolerability, efficacy & PK of PT217 for treating patients with advanced or refractory cancers expressing DLL3
- PT217 is a native IgG-like bispecific Ab that works by targeting DLL3 and CD47 and is under development for small cell lung cancer (SCLC), large cell neuroendocrine carcinoma of the lung (LCNEC) and extrapulmonary neuroendocrine carcinomas (EP-NECs)
 Diazoxide Choline
Diazoxide Choline

- The US FDA has granted BTD to diazoxide choline to treat Prader-Willi syndrome (PWS) in adults and children ages 4yrs. and older with hyperphagia, supported by the P-III study to evaluate its effectiveness
- The drug is also designated with ODD across the US & the EU along with FTD across the US
Ziftomenib
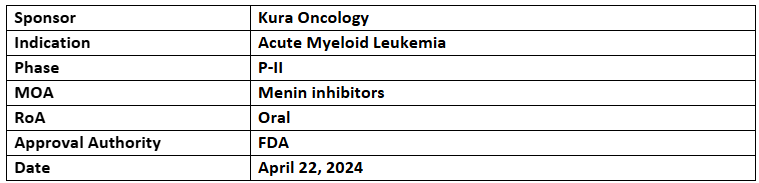
- The ziftomenib has been designated with BTD to treat r/r NPM1-mutated AML, supported by the company’s ongoing KOMET-001 study
- The company anticipates the completion of KOMET-001 by mid-2024
- Ziftomenib further being assessed in KOMET-007 combined with venetoclax/azacitidine or cytarabine + daunorubicin (7+3) as well as in KOMET-008 in combination with gilteritinib, FLAG-IDA or LDAC for treating NPM1-mutant & KMT2A-rearranged AML
Sunvozertinib
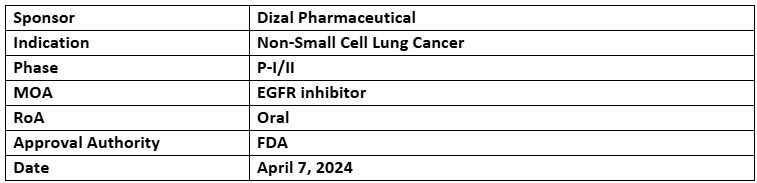
- The BTD has been granted to sunvozertinib for the 1L treatment of locally advanced or metastatic NSCLC associated with EGFR exon 20 insertion mutation
- The designation was supported by the P-I/II (WU-KONG1) study depicting cORR of 78.6% and mPFS of 12.4mos. Results were highlighted at ESMO 2023
- The drug is being assessed under 2 studies as ≥2L (WU-KONG1 PART B) and 1L (WU-KONG28) for the same indication
- The company anticipates the NDA submission across the US & the EU later in 2024.
- Sunvozertinib was previously granted BTDs by both the US FDA and the China CDE for relapsed or refractory patients and approved by China CDE in 2023 for 2L patients.
 Fabhalta
Fabhalta
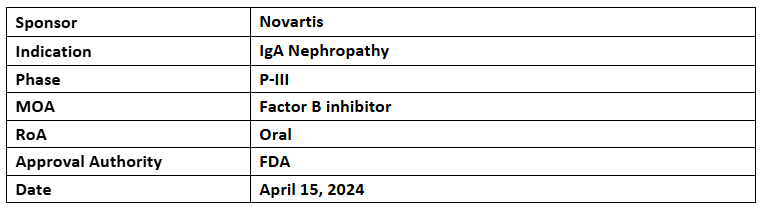
- Novartis has released pre-specified interim analysis data from the P-III (APPLAUSE-IgAN) trial to investigate Fabhalta's (200mg, oral, BID) safety & efficacy vs PBO to treat primary IgAN adults (n=518). The analysis evaluated 250 participants for efficacy & 443 for safety
- The analysis demonstrated a 38.3% reduction in proteinuria at 9mos. vs PBO alongside supportive care. The 1EP of slowing IgAN progression, estimated by annualized total eGFR slope at 24mos. is anticipated during trial completion in 2025
- The FDA accepted submission for accelerated approval and granted priority review status
- The safety of the drug was well-tolerated and favorable, aligning with the prior studies. These results plus IgAN & C3 glomerulopathy (C3G) real-world studies were highlighted at WCN
Jemperli (dostarlimab)
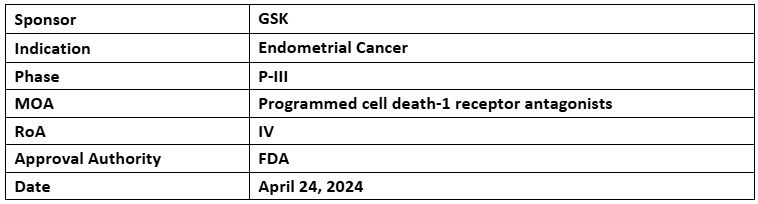
- The US FDA has accepted and granted priority review to the sBLA of Jemperli (dostarlimab) + CT (carboplatin and paclitaxel) for treating primary advanced or recurrent endometrial cancer adults with MMRp/MSS tumors. The decision is anticipated on Aug 23, 2024
- The sBLA was supported by part 1 data of the P-III (RUBY) study assessing dostarlimab + CT followed by dostarlimab vs PBO + CT followed by PBO; part 2 of the study assesses dostarlimab + CT followed by dostarlimab + niraparib vs PBO + CT followed by PBO for treating primary advanced or recurrent endometrial cancer
- Part 1 of the study met the 1EPs of PFS & OS, showing advantages in the overall population with a tolerable and consistent safety profile. OS data was highlighted at SGO 2024
Fruquintinib
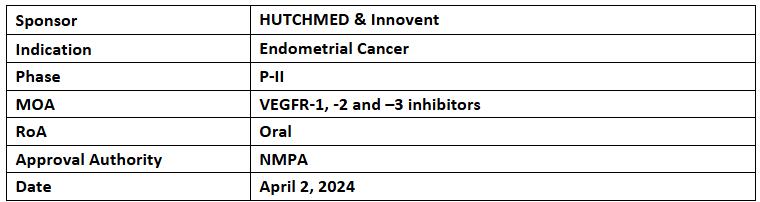
- The NMPA has accepted & granted priority review to the NDA of fruquintinib + sintilimab for treating advanced endometrial cancer patients having pMMR1 or non-MSI-H2 tumors, were failed on previous systemic therapy & are not eligible for curative surgery or radiation
- The NDA was based on the endometrial cancer registration arm, FRUSICA-1, of P-II study assessing fruquintinib + sintilimab to treat endometrial cancer patients having disease recurrence & progression or intolerable toxicity due to Pt-based doublet CT with the 1EP as ORR & the 2EPs as DCR, DoR, PFS, OS & PK characteristics
- Additionally, in Jul 2023, the NMPA granted BTD to fruquintinib combined with sintilimab for the same indication
 SGT-003
SGT-003
- SGT-003 (1E14vg/kg) is being assessed in the P-I/II (INSPIRE Duchenne) study for its safety & tolerability to treat DMD pediatric patients across 2 arms; arm 1 enrolls patients having 4 to <6yrs. and arm 2 enrolls patients 6 to <8yrs. of age
- The safety results from first 3-4 patients are anticipated in mid-2024 with initial expression and functional data in Q4’24
- SGT-003 utilizes a capsid (AAV-SLB101) to transfer a DNA sequence encoding microdystrophin with the R16-R17 nNOS binding domain
 Acolyte Image Guided Crossing and Re-Entry Catheter System
Acolyte Image Guided Crossing and Re-Entry Catheter System
- The US FDA has granted breakthrough device designation to the company’s Acolyte Image Guided Crossing and Re-Entry Catheter System for treating coronary chronic total occlusions (CTOs)
- The Acolyte system positions guidewires and catheters properly into the coronary vasculature to treat coronary CTOs patients whose symptoms persist post-medical therapy
- The device further offers real-time optical coherence tomography (OCT) visualization & navigation capabilities for placing guidewires and catheters into the target vessel's true lumen enabling CTO crossing to improve surgeries
Elecsys pTau217 Assay
- The US FDA has granted breakthrough device designation to the company’s blood test, Elecsys pTau217 assay, for the diagnosis of Alzheimer's disease
- Developed in partnership with Eli Lilly, the assay is intended to detect the presence or absence of amyloid pathology in individuals enabling appropriate treatment through participation in clinical evaluations or accessing approved disease-modifying therapies
- The Elecsys Phospho-Tau (217P) is an in vitro immunoassay developed for the determining Phospho-Tau (217P) protein quantitatively among individuals of age 60yrs. & above and is intended to diagnose Alzheimer's disease
JuxtaFlow Renal Assist Device (RAD)
- The designation was based on the data from BIPASS-AKI feasibility study assessing the safety and performance of the JuxtaFlow System in individuals with pre-existing renal insufficiency who are undergoing cardiac surgery
- The device works by putting a gentle negative pressure on the kidneys' urine-collecting system to improve its function and protect against hypoxia-induced damage in acute conditions
- Furthermore, its US launch is expected in late 2025 with its application expansion beyond cardiac thoracic surgery
 rAAV-Olig001-ASPA
rAAV-Olig001-ASPA
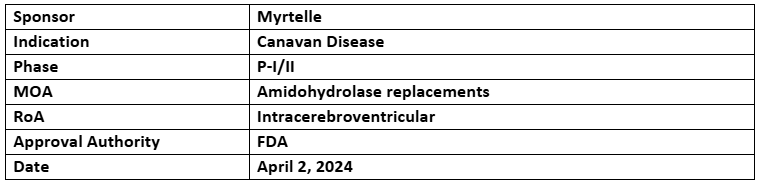
- The designation was supported by the preliminary data showing the drug’s potential to treat the disease
- The company is evaluating rAAV-based vector, rAAV-Olig001-ASPA, under the P-I/II study to address Canavan Disease (CD)
- rAAV-Olig001-ASPA has already received ODD, RPDD, FTD from US FDA, ODD, ATMP from EMA and ILAP from UK MHRA in past
References:
- Lisata Therapeutics PR
- BiVictriX Therapeutics PR
- Candel Therapeutics PR
- Moleculin Biotech PR
- Immix Biopharma PR
- Edgewise Therapeutics PR
- Phanes Therapeutics PR
- Telix PR
- Syros Pharmaceuticals PR
- AskBio PR
- PureTech PR
- Lexeo Therapeutics PR
- AdrenoMed PR
- Soleno Therapeutics PR
- Kura Oncology PR
- Solid Biosciences PR
- Myrtelle PR
- Roche PR
- HUTCHMED PR
- Novartis PR
- GSK PR
- Businesswire
- PR Newswire
- Globe News Wire
Related Post: New Drug Designations - March 2024
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














